
It is a method of expressing the composition of a mix with a dimensionless quantity, the mass fraction (percentage by weight, wt%) and volume fraction (percentage by volume, vol%). For mixtures of gases, IUPAC recommends the letter y.

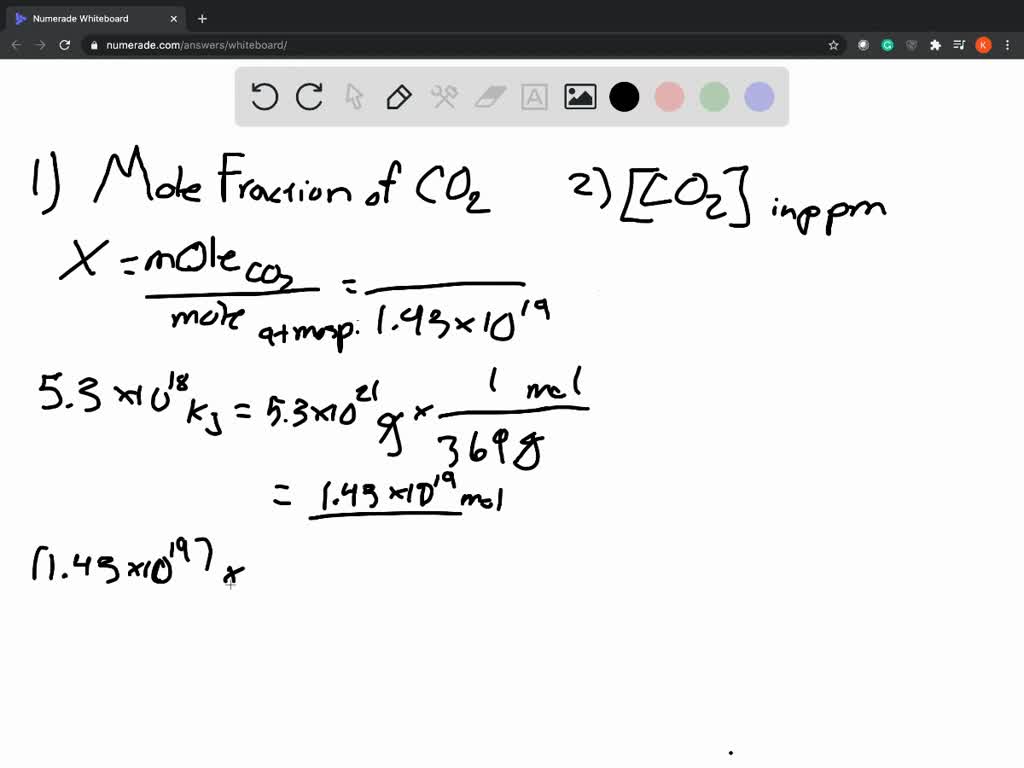
The lowercase Greek letter χ (chi) rather than a Roman x are the notations of mole fraction. Mole fraction of every solute = number of moles of that solute/ the entire number of moles of all solutes and also the solvent. During this article, we’ll discuss the mole fraction formula, its properties, and solved examples. Since the quantity fractions are like mole fractions, the mixture is additionally 90%, by methane. For instance, if the mole fraction of methane in gas is 0.90, then this suggests that 90% of the molecules are methane. A mole fraction is the ratio of molecules of 1 component during a mixture. A mole is really a variety of molecules, approximately 6 × 10 23. Water hardness can be measured in gpg or ppm, but gpg is the most commonly used measurement.Mole fraction may be a unit of concentration in chemistry. One grain per gallon (gpg) is equivalent to 17.14 parts per million (ppm). One ppm is equivalent to 1 milligram of something per liter of water (mg/l) or 1 milligram of something per kilogram soil (mg/kg). Usually describes the concentration of something in water or soil. This conversion works for all nutrients reported in these units: macronutrients, such as calcium or magnesium, and micronutrients, such as iron, manganese, zinc, and copper. To convert 120 pounds per acre to ppm, just multiply 120 pounds per acre by 0.5, which is to equal 60 ppm. How do you convert pounds per acre to ppm? Parts per million: The number of milligrams of solute per kg of solution = one ppm, since 1 mg = 10. Concentrations expressed as ppm and N are less familiar to most students at this stage. Mole fraction, χ = moles of solute/total moles. Molar concentration (moles per volume) Mass per volume (sometimes called ppm)…Mol Fraction. Just multiply g/L by 1000 to convert g to mg, and you have ppm (in mg/L of water). If you take molarity (with units mol/L), and multiply it by the molar mass (with units g/mol), you get g/L. To convert ppm to molarity, or molarity to ppm, you only need to know one thing: the molar mass of the dissolved element or molecule. How do you calculate molar concentration from ppm? Therefore, it is common to equate 1 kilogram of water with 1 L of water. When working with aqueous solutions, it is common to assume that the density of water is 1.00 g/mL. The quantity “1 ppm” can be used for a mass fraction if a water-borne pollutant is present at one-millionth of a gram per gram of sample solution. Just as per cent means out of a hundred, so parts per million or ppm means out of a million. Both parts of the equation must be in the same format, weight or volume. How do you calculate ppm? PPM is calculated by dividing the mass of the solute by the mass of the solution, then multiplying by 1,000,000. Assume the solute is sodium chloride ( Mr=58.44 ). Here, we must know the molar mass of the solute.


 0 kommentar(er)
0 kommentar(er)
